Chlorine analyzers from Pi are used in many applications requiring the measurement and control of online residual chlorine levels in water. The HaloSense range is suitable for total or free residual chlorine monitoring or control applications in potable water, seawater, process water, swimming pool water, waste water, food washing, paper and pulp, etc.
The following are available in the HaloSense range;
- Online free chlorine 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm, 0.5-200ppm
- Online total chlorine 0.005-0.5ppm, 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm
- Online residual chlorine in seawater analyzers (free or total bromine) 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm
- Online zero chlorine (designed to measure the absence of free chlorine) 0.005-2ppm for applications such as post activated carbon and pre-RO monitoring.
The HaloSense range of controllers/transmitters means that you get exactly what you need and nothing that you don’t. From a low cost no-frills chlorine dosing controller (CRONOS®) to a highly sophisticated colour display, remote access controller (CRIUS®) – and all with the same great sensors! Chlorine dosing control is now simpler and cheaper than ever! Both instruments can have multiple sensors and multiple sensor types, saving money on the requirement for one sensor and one transmitter per measurement.
The membraned amperometric sensors are enhanced with a third, reference electrode which eliminates zero drift. (NB. These chlorine sensors are often known as polarographic sensors although this is a misuse of the word polarographic). Its unique design means that pH compensation is not usually required at all, completely eliminating reagents.
The free chlorine sensors used by the analyzers are largely pH independent meaning that the measurements are bufferless and reagentless. They are amperometric sensors and show remarkable sensitivity and stability. For those needing to measure chlorine at high pH (>pH 8.5) on variable pH water it is possible to provide pH compensation from either a pH sensor connected to the transmitter or from an external pH meter.
The sensors work by separating the electrodes that perform the measurement from the sample, by a membrane. This membrane allows the free residual chlorine (HOCl and OCl–) or the total residual chlorine (HOCl and OCl– plus chloramines) through the membrane. Inside the sensor the dissolved chlorine meets the electrolyte which is at a low pH. This converts the majority of the OCl– to HOCl. The HOCl is reduced at the gold working electrode and the current generated is proportional to the chlorine present, and the instrument gives a reading in ppm or mg/l.
This technique is the most advanced method of continuous chlorine measurement and has many benefits to the user including a very stable online measurement and better dosing control.
The HaloSense range is bufferless and reagent free, meaning that it has a low total cost of ownership and with maintenance intervals of 1 year. HaloSense is fast becoming the instrument of choice for the engineer who wants the best instrument at the best price.
- Low purchase cost
- Low cost of ownership
- Reduced pH dependency (largely pH independent)
- Stable and reliable
- Bufferless
- Reagentless
Many water companies want to measure free chlorine residuals without the need for chemical buffers traditionally associated with such measurements. Acetate and phosphate buffers are expensive and environmentally unfriendly. Buffer delivery systems are maintenance intensive and have fairly costly consumables and there are health and safety considerations in the handling of the acids and high disposal costs if the acid treated water is unable to be fed back into the water supply.
Amperometric cells and most polarographic probes only respond to hypochlorous acid, (HOCl). HOCl dissociates into hypochlorite (OCl–) in a pH dependent manner. This is why most chlorine monitors need acid buffers in most applications. The typical pH of water measured on a water treatment works may range from 7 to 9.2. Chemical buffering reduces the pH to between 5 and 6 and ensures that the majority of the residual chlorine is present as HOCl (see graph below).
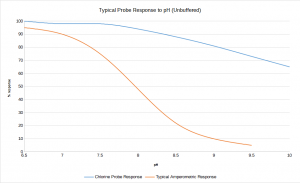
No Buffer or pH Compensation Required Needed With The HaloSense
The HaloSense Free Chlorine Sensor measures all the HOCl and the majority of the OCl– present (blue line on graph). This results in a vastly reduced pH effect and means that most chlorine monitoring applications require no buffer and no pH compensation.
Need help with an application? Click here!
- Continuous online monitoring for residual chlorine in any water
- Water treatment plant residual chlorine dosing control
- Secondary chlorination free chlorine dosing control
- Distribution monitoring
- Cooling tower monitoring and control
- Pasteurizer dosing control
- Seawater chlorination control
- Bromine monitoring in seawater
- Food washing
- Chloramination control
The HaloSense chlorine monitor range is particularly suited to working in sites where reliability and ease of use are most important.
Possibly the best Chlorine Monitor in the world – the CRONOS® HaloSense in the Distribution System.
Can the CRONOS® HaloSense be the ‘best’ Chlorine Monitor in the world?
It depends entirely on what you mean by ‘best’ but it has to be up there as a strong contender.
What do we need from a chlorine monitor on the distribution network?
- Specificity
- Low drift (high accuracy)
- Low maintenance (and low maintenance cost)
- Long intervals between maintenance
- Long intervals between calibration
Every company that sells total or free chlorine monitors will claim these attributes for their product but have they done the research?
In 2009 a customer bought 334 chlorine monitors from Pi for both drinking water chlorination control (64 instruments) and distribution monitoring (270 instruments). They did this after a year’s trial of a range of analyzers available on the market. In 2012 they did a second trial on 30 of the distribution chlorine monitors installed in order to prove the savings that they had made.
They effectively ran the monitors until they failed without re-calibration and without maintenance. The findings of the research was that the HaloSense could
“be within 10% of the calibrated value for more then 90 days without ANY operator/ technical intervention (i.e no calibration and no maintenance).”
Does that make the CRONOS® HaloSense the best chlorine monitor in the world? Maybe, but coupled with its on-board control options, its lack of response to the changing pH conditions in the distribution system, its suitability for free chlorine and total chlorine monitoring applications, its comms capability and its remarkably low total cost of ownership, it could be the ‘best’ chlorine monitor in the world.
For more information on the remarkable CRONOS® HaloSense, installed in over 40 countries around the world please contact us.
Treating and disinfecting potable water at a water treatment facility are only some of the problems associated with ensuring that drinking water reaches the taps safely. In long distribution networks it is necessary to monitor for a chlorine residual and if necessary to top it up with a small secondary chlorination plant. In order to maintain a residual all the way to the taps.
Secondary chlorination plants, by their nature are often unmanned and in remote, inaccessible locations. For these reasons a chlorine meter/chlorine controller/chlorine analyzer should have the following benefits:
- Upto a year between maintenance
- Three months between calibration
- Unaffected by changes in the pH found in distribution
- Low capital cost and cost of ownership
- No buffers or reagents
In 2012/2013 extensive tests on the Pi residual chlorine analyzer (HaloSense) on 30 chlorine measurement instruments installed in Ireland showed that the chlorine meters achieved all the above and proved themselves in what can be a very demanding application. Click here to contact us for more information on this and other chlorine measurement applications.
What do water companies really want when they buy a chlorine analyzer to monitor the chlorine residuals in their distribution system?
They want three things above all others.
1) Simple
- No reagents
- No special sample disposal requirements
- Small mounting footprint
- Single point calibration
2) Reliable
- Comprehensible drift between calibration and maintenance
- Up to three months between calibration
- Up to six months between maintenance visits
3) Cost Effective
- Low capital cost
- Low installation cost
- Low cost of commissioning
- Low cost of ownership
- Up to three months completely unattended operation!
All over the world customers have done side by side trails against other analyzers and in the majority of cases they find that the Pi HaloSense sensors for free chlorine and total chlorine provided the most simple, reliable and cost effective solution to measuring the chlorine residuals in their distribution systems.
 Small water treatment plants, secondary disinfection plants etc. tend to suffer from similar problems wherever they are in the world. The first is lack of communications SCADA infrastructure, the cost of which to install can be prohibitive. The second is the lack of a central DCS control infrastructure, again the installation of which can be cost prohibitive. The third is the remote location. Often these water treatment plants are in remote and difficult to access locations.
Small water treatment plants, secondary disinfection plants etc. tend to suffer from similar problems wherever they are in the world. The first is lack of communications SCADA infrastructure, the cost of which to install can be prohibitive. The second is the lack of a central DCS control infrastructure, again the installation of which can be cost prohibitive. The third is the remote location. Often these water treatment plants are in remote and difficult to access locations.
With these three issues facing many water engineers around the world, a low cost solution providing solutions to all three issues is available from Pi. A CRIUS® controller has the on board capacity to provide small scale SCADA, and full online PID control whilst the sensors (e.g. chlorine, pH, Turbidity etc.) are suitable for long term operation without operator intervention.
To demonstrate the remote access capability of the CRIUS® please click here.
To learn more about the control capability of the CRIUS® please click here.
To hear more about other customers using the CRIUS® multiparameter controllers in a similar way why not contact us?
Pasteurizers and their disinfection control pose particular problems for online instruments. Most online instruments require a continuous sample whereas a pasteurizer is more usually on and off as required.
Also the contents of a pasteurizer are not as gentle as drinking water, often at an elevated temperature with fluctuating pH, and the presence of detergents (tensides).
Pi has been working with a few household names to provide a packages solution to these problems and now has several installed across the UK on cider and beer bottler plants. The sensor is housed on a dual purpose autoflush device which can not only keep the sensor clean but also keeps it wet when the process is off.
Also providing remote access, email alarms, text alarms and full PID control the Pi pasteurizing disinfection control systems are suitable for chlorine, bromine, ozone and chlorine dioxide disinfection with specific sensors for each.
Why not contact us and see what we may be able to do to help with your pasteurization application.
The HaloSense sensors can come equipped to automatically clean themselves at user defined intervals, with all the benefits of no operator intervention for up to 6 months. The autoflush is particularly useful in food preparation, pulp and paper, and many applications where there is likely to be a build up of solids in the sample. For more information about autoflush click here.
Get more info on the Residual Chlorine Analyzer
Please contact us by using the form below.
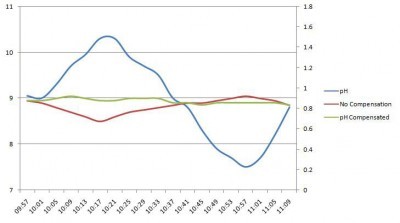 For some free chlorine applications with high and variable pH, pH compensation can improve the accuracy of the analyzer. For pH compensation to be valid it must be done with the highest quality pH sensors and with chlorine sensors that have a reduced susceptibility to varying pH, such as those used in the HaloSense range.
For some free chlorine applications with high and variable pH, pH compensation can improve the accuracy of the analyzer. For pH compensation to be valid it must be done with the highest quality pH sensors and with chlorine sensors that have a reduced susceptibility to varying pH, such as those used in the HaloSense range.
The graph shows the errors on a real HaloSense free chlorine sensor when a sample of 1 ppm free chlorine has the pH changed from pH 9 to more than pH 10, down to pH 7.5 and back again. The graph shows that the vast majority of applications won’t need pH compensation at all and for those that do that free chlorine sensor is the most appropriate sensor available to have that compensation applied.
Each Residual Chlorine Analyzer from Pi has the capability to be an extremely capable Chlorine Controller. The controllers can have multiple control channels which can utilise chemical control (usually a relay (switch) turns dosing on when the chlorine is too low or off when it is too high) or PID control.
PID stands for Proportional Integrated Derivative and it is a mathematical manipulation of the sensor signal to give an output that will control a pump and manage a constant chlorine level in the water. All the features are adjustable and there are safety features built in such as overfeed protection. For a discussion of PID control please see our technical notes here.
Pi’s chlorine controllers have been used in many control applications such as in pasteurizers, water treatment, cooling towers, swimming pools etc.
| Document | Type | Size |
|---|---|---|
HaloSense |
Brochure | 586kB |
pH Compensation |
Technical Note | 414kB |
pH Effects on Pi’s Free Chlorine Sensor |
Technical Note | 492kB |
Free Chlorine Probe Maintenance |
Technical Note | 556kB |
Total Chlorine Maintenance |
Technical Note | 556kB |
Seawater Chlorination |
Technical Note | 558kB |
HaloSense Hints and Tips |
Technical Note | 451kB |
HaloSense Zero |
Technical Note | 612kB |
ORP vs. ppm |
Technical Note | 512kB |
CRONOS® |
Brochure | 582kB |
CRIUS® |
Brochure | 584kB |
CRIUS® Remote Communications |
Brochure | 573kB |
CRONOS® and CRIUS® Control Options |
Technical Note | 534kB |
Remote Access GPRS |
Technical Note | 481kB |
Autoflush |
Brochure | 434kB |
Probe Fouling |
Technical Note | 382kB |
When chlorine is added to pure water between pH 4 and pH 11
Cl2 + OH– ↔ HOCl + Cl–
HOCl ↔ OCl– + H+
so if chlorine is added to water you get HOCl (Hypochlorous acid) and OCl– (Hypochlorite), which together make ‘free chlorine’.
NH3 + OCl– → NH2Cl + OH–
In an acidic solution Monochloramine disproportionates to form Nitrogen Trichloride.
2NH2Cl + H+ → NHCl2 + NH4+
3NHCl2 + H+ → 2NCl3 + NH4+
In solution where there are low concentrations of chlorine it is often Chloramines that can be smelled not ‘chlorine’.
The three Chloramines above are collectively known as ‘Combined Chlorine’.
Focus Ons are a series of short articles distributed by email providing technical information regarding instrumentation, process measurement in potable, waste, process and pool waters. If you would like to join the mailing list, please contact us.
You probably know that some instruments use ORP to control chlorine dosing and others use ppm chlorine sensors but…
… did you know that ORP over about 3 ppm won’t work?
… did you know that swimming pools in the USA use ORP and in Europe use ppm chlorine sensors?
… that the ORP of towns water can vary a great deal?
In the USA nearly all pools and spas use ORP sensors to control their chlorine dose, yet conversely in the UK and Western Europe most ORP systems have been replaced with systems that measure the concentration of free chlorine in water. Pi provides systems that utilize either or both technologies.
ORP
Oxidation reduction potential (ORP or REDOX) sensors, measure the tendency of water to gain or lose electrons from anything in the water. The more positive a reading from an ORP the greater the tendency the water has to oxidize (gain electrons from) organisms or other material in the water, thereby killing or destroying them.
Why do so many pools in the USA use ORP?
When chlorine is dosed into a pool it form OCl– and HOCl. Disinfection is largely done by the HOCl and ORP responds to the concentration of HOCl in the water, which makes it a good measure of the tendency of the chlorine in the water to kill bugs. Despite this, ORP is a secondary measure of HOCl and is affected by a multitude of other factors, some of which will be touched on below. The main attractions of ORP are; low purchase cost, no calibration and little or no maintenance.
What are the problems with ORP sensors?
Unfortunately, what ORP sensors measure is tendency and not capacity, i.e. ORP measures the likelihood or the ability of the water to kill bugs, but not how many bugs that water can kill, a subtle but very important difference. A sample with high ORP may be able to kill a small number of bugs very quickly but then not be able to kill future pollution. What’s more, although chlorine affects ORP very strongly it is not the only variable involved. The pH of water affects ORP directly and also affects the concentration ratio of OCl–/HOCl, the two main disinfectant components. A lower pH (higher acidity) will cause an increase in the relative concentrations of HOCl causing an increase in ORP.
Perhaps the biggest issue with ORP is that the ORP readings on water with no chlorine in it will be different depending on the source of that water. This means that an ORP of 750mV in one part of the country is not the same chlorine concentration as 750mV in another part of the country. Also the ORP response to HOCl is not linear and increasing residual chlorine above 3 ppm has little effect on ORP readings making control above 3 ppm extremely difficult. These issues typically lead to overdosing the water with chlorine, in order to compensate for these effects. This can be seen very clearly in US pools which often have more than 2 ppm of chlorine compared to European pools which typically operate around 0.8-1.5 ppm (The World Health Organization recommends 1 ppm residual).
ppm Chlorine
These sensors use electrochemistry to measure the free chlorine concentration directly. They tend to be slightly more expensive than an ORP sensor, but are more reproducible and precise, and therefore tend to give better control (and therefore reduced chemical cost). They are specific to free chlorine (the disinfectant) and can be easily calibrated using a DPD test for free chlorine. Whilst the capital cost for a ppm chlorine sensor is higher, total cost of ownership tends to be lower as ORP sensors are typically replaced every year and ppm sensors last for ten years or more.
Problems with ppm Chlorine sensors
A ppm sensor measures the capacity of water to kill organisms, the only problem is that it doesn’t measure how fast the bugs are killed, a variable largely down to pH. There are two different types of ppm sensors. The first measure only HOCl, and have very similar problems to ORP sensors. The other type of sensor, in pHs below 8.0, measure both HOCl and OCl–. Pi only recommends the use of sensors that (for use in pools) are independent of pH, and the use of pH control that is independent of chlorine dosage. This leads to tighter control of both pH and free chlorine meaning chlorine residuals can be more tightly controlled and reduced, which in turn leads to lower costs and a more pleasant bathing experience.
Conclusion
| Advantages | Disadvantages |
|---|---|
ORP SensorsSimple (no calibration) |
ORP SensorsDoesn’t measure disinfection capacity |
ppm SensorsMeasure free chlorine directly |
ppm SensorsRequires calibration |
Did you know that when you dose chlorine into seawater it is bromine that does the disinfection?
Did you know that DPD 1 measures free chlorine or total bromine and not free bromine?
Chlorination Chemistry of Seawater
The chemistry of the chlorination of seawater is more complex than many people realise and although the measurement of chlorine residuals is possible in seawater (and therefore automatic control of chlorine dosing), better results will be obtained if this is fully understood.
Is it Chlorine or is it Bromine?
Seawater contains about 70 ppm dissolved bromides most of which are sodium bromide. When you put chlorine in water it displaces (because it’s more reactive) the bromine from the bromide and becomes a chloride. So for up to about 70 ppm of total chlorine dosed what you actually have in the water is free bromine and combined bromine (NOT free and combined chlorine) so it is the total bromine that actually does the disinfection [1]. So why does everyone call it chlorination when technically it is bromination? Mainly because most people don’t know this interesting bit of chemistry. So what? Normally it makes no difference at all as bromine is an effective disinfectant, however there can be a lot of confusion when it comes to monitoring residuals and controlling dosing. Choosing the correct sensor to control the dosing is crucial as is choosing the correct DPD test.
Pi offer a specialist range of seawater chlorination controllers, but to choose the right controller we need to understand the chemistry going on. A technical note on the same subject is available here.
Free Chlorine and Total Bromine
 Due to the confusion on what is being measured it is easy for an engineer to specify the wrong equipment and calibrate it incorrectly. For example, it is common for a free chlorine sensor to be specified for seawater chlorination control. Most electrochemical free chlorine sensors will react to free bromine (not all so be careful!) but this isn’t necessarily what you need for bromination control. Most authors agree that whilst the disinfection capability between free chlorine and combined chlorine differs, when it comes to free bromine and combined bromine, both forms of the chemical are equally good at disinfection so a better measurement would be total bromine, which requires a total bromine sensor.
Due to the confusion on what is being measured it is easy for an engineer to specify the wrong equipment and calibrate it incorrectly. For example, it is common for a free chlorine sensor to be specified for seawater chlorination control. Most electrochemical free chlorine sensors will react to free bromine (not all so be careful!) but this isn’t necessarily what you need for bromination control. Most authors agree that whilst the disinfection capability between free chlorine and combined chlorine differs, when it comes to free bromine and combined bromine, both forms of the chemical are equally good at disinfection so a better measurement would be total bromine, which requires a total bromine sensor.
DPD and Seawater Chlorination
To add to this already confusing environment we need to look at calibrating online sensors or using handheld photometers to track the residual. DPD is used extensively to measure chlorine residuals and it also reacts to bromine so can be used for both, however, DPD 1 measures FREE chlorine or TOTAL bromine. The situation can therefore arise where you have an online instrument such as a CRONOS® or CRIUS® specified as a free chlorine, actually measuring free bromine but calibrated as a total bromine (against DPD 1)! Typically the best results are obtained by specifying a total bromine (total chlorine) sensor and calibrating it using DPD 1. That, however, isn’t the end of the story! When specifying an analyzer it is crucial that we suppliers know that it is for use with seawater because the physical and chemical make-up of seawater is very different to potable or process water and this can affect what we would supply to customers.
The effect of Salinity on Membrane Sensors
It is crucial for us to know if you are going to use a Pi sensor in seawater so we can provide you with a saltier electrolyte. Osmosis means that water moves from a low solute concentration to a higher solute concentration across a semi-permeable membrane. The electrolyte in our sensors is saltier than potable or process water so osmosis forces water into the end of the sensor, which the sensor is designed to cope with, however, with seawater the process is reversed and the water in the electrolyte can be forced out of the sensor into the sample. To solve the problem we supply electrolyte especially designed for seawater, with a higher salinity.
Estuarine Waters
Many seawater chlorination applications are estuarine in nature (partly seawater and partly fresh water) and it is the degree of dilution which determines which sensor and which electrolyte you should use. Seawater has approximately 70 ppm bromides and so up to 70 ppm chlorine the replacement will be 100%. If the seawater is 50% fresh water then up to 35 ppm chlorine will give 100% displacement. For example, if we looked at a 2 ppm residual then the water could be only 3% seawater and 97% fresh water and you would still be measuring bromine, so a total bromine sensor calibrated with DPD 1 would be appropriate. For any water that is contaminated with seawater the seawater electrolyte is likely to be the most appropriate.
The Solution!
If all of this is too much to take in and remember, then don’t worry! Just remember to talk to Pi for any online chlorination application and we will do the rest… guaranteed!
References
[1]. White’s Handbook of Chlorination and Alternative Disinfectants, 5th Edition, Wiley – page 874, pages 122-129.
… Pi has a security system that can alert you to any free chlorine present and can actually prevent the contaminated water from reaching the membranes at all?
The most common source of water for corporate buildings, hospitals and industrial processes, is standard mains water. This water typically contains chlorine, added by municipal water companies, for its disinfection properties. For any system that requires RO membranes, if the water source is mains water, it is common to have carbon filters positioned before the membranes. The carbon filters are designed to remove any chemicals in the water that might be damaging to the RO membranes, including chlorine.
 Our HaloSense Zero chlorine controller measures the water coming out of the carbon filters before it reaches the RO membranes. Any free chlorine breakthrough from the carbon filters is detected by the system, which will set off an alarm and can also be configured to automatically shut off valves to prevent the contaminated water from reaching the RO membranes.
Our HaloSense Zero chlorine controller measures the water coming out of the carbon filters before it reaches the RO membranes. Any free chlorine breakthrough from the carbon filters is detected by the system, which will set off an alarm and can also be configured to automatically shut off valves to prevent the contaminated water from reaching the RO membranes.
The HaloSense Zero effectively acts as a security measure to ensure that if there is a breakthrough of chlorine, the system can be shut down to prevent damage to the RO membranes. This can help to extend the lifetime of the expensive RO membranes and save the customer money.
The removal of chlorine is even more important in hospital systems where the water is supplied to renal wards. In this case the HaloSense Zero chlorine analyzer not only protects the RO membranes, but it can also help to save lives. If any chlorine was to break through the carbon filters and make it past the RO membranes as well, it could endanger the lives of renal patients in the hospital. The HaloSense Zero chlorine system is a fantastic safety feature, providing an extra line of defense against the potential hazard.
 The HaloSense Zero comprises of a sensor designed to measure the absence of chlorine connected to a CRONOS® or CRIUS® controller. The sensor can detect even very low levels of free chlorine, while the CRONOS® or CRIUS® controller acts as the brains of the system, interpreting data from the sensor. In the event that any free chlorine is detected, the analyzer will send an alarm signal and can automatically shut off valves to prevent chlorine from reaching the RO membranes.
The HaloSense Zero comprises of a sensor designed to measure the absence of chlorine connected to a CRONOS® or CRIUS® controller. The sensor can detect even very low levels of free chlorine, while the CRONOS® or CRIUS® controller acts as the brains of the system, interpreting data from the sensor. In the event that any free chlorine is detected, the analyzer will send an alarm signal and can automatically shut off valves to prevent chlorine from reaching the RO membranes.
So why aren’t you using this system already? Are there any catches?
The only catch is that the HaloSense Zero can’t measure combined chlorines in the water. Typical mains water in most countries contains a mixture of free chlorine and combined chlorine which is what the HaloSense Zero is designed to detect and help protect the RO membranes against.
The ingenious HaloSense Zero chlorine analyzer will even periodically check the responsiveness of the sensor automatically, to ensure that the sensor is still reacting correctly when free chlorine is present. It does this by switching between the post-carbon filter water and chlorinated mains water using a 3-way solenoid valve controlled by a programmed timer.
If you are using a carbon filter before your RO membranes to remove the chlorine, probably the only reason you aren’t using this system already is simply that you haven’t heard of it before. The Pi HaloSense Zero chlorine system is just one of Pi’s innovative solutions to water treatment problems.
You probably know that most chlorine, ozone and chlorine dioxide analyzers are calibrated using hand held DPD kits but…
… did you know that DPD can’t tell you when you have no residual?
… did you know that errors on DPD performance can be up to ± 100%?
… did you know that a significant number of service calls received by Pi relate to poor calibration?
DPD (N.N-diethyl-p-phenylenediamine) is a chemical that when mixed with water containing an oxidant, changes colour depending on the concentration of the oxidant present. A handheld colorimeter measures light passing through the coloured solution. The absorption of that light by the liquid gives a concentration value. It is usually used to check concentration of, for example, free chlorine, total chlorine, ozone and chlorine dioxide etc. in water.
When the DPD kit gives a value, it is often used to calibrate online instruments……and that is where Pi comes in!
As a manufacturer of online instruments we have to understand DPD in order to help our customers when they have problems calibrating their online monitors.
This Focus On will look at:
- The limitations of DPD (turbidity, zero oxidant, bleaching, pH and interferents).
- Minimizing DPD measurement error (sampling, alignment and cleaning).
- Things to look out for (low concentrations, pink color, stained glass).
- Little known chemistry (measuring bromine, chlorite versus chlorine dioxide).
- Rinse and repeat: is it really worth repeating my measurement?
What are the limitations of DPD?
DPD cannot measure zero oxidant well.
DPD works using the absorption of light, and turbidity in the sample will give a positive reading. This means if there is no oxidant in the sample, any turbidity introduced to the sample after ‘zero’ such as undissolved tablet or powder will cause the DPD test kit to give a small reading, this is why…
DPD cannot measure below approximately 0.05 ppm.
If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink color, it is likely any reading you are getting is from the unreacted DPD tablet.
DPD cannot measure free chlorine above 6 ppm
Many people are unaware that past a certain level of oxidant, DPD will not form its characteristic pink color, and instead will ‘bleach’ to form a clear solution. This can lead people to think there is little or no oxidant in their water, when in fact there is so much that it is bleaching their DPD. Be on the lookout for a flash of pink when the tablet or powder is added if you suspect your sample is being bleached. NB. special kits and reagents are available for measuring oxidant above 6 ppm.
DPD cannot measure in extremes of alkalinity or pH.
DPD tablets, powdered pillows, and drops contain buffers that will change the pH of your solution in order to facilitate DPD reacting with your oxidant. There is only so much buffering capability in the powder or tablet, and if your sample has an extreme of pH or alkalinity this could affect the concentration reading from the DPD handset.
DPD cannot distinguish between oxidants such as:
chlorine, chlorine dioxide, chlorite, ozone, organochlorides, bromine and more, meaning interferents are a big problem.
DPD is a fantastic chemical, in that it is very versatile as a coloring agent, which is how it gives the oxidant the colour that we measure. This versatility does come at a price, DPD is not very specific as an analysis tool, and so if other chemicals are present in the sample, they can interfere with the reading, giving an inaccurate result. Common interferents include chlorine dioxide (for chlorine measurement, and vice versa), sodium chlorite, ozone, organochloramines, peroxides, and many more.
DPD cannot distinguish between color and turbidity.
Any undissolved solids, including unreacted DPD tablet, will affect the reading. Sample turbidity should be accounted for in the zero measurement. If the zero measurement has a high turbidity, this will affect the sensitivity of the colorimeter, due to the large correction it must perform to account for absorption by undissolved solids. Allowing any solids in the sample several seconds to settle after mixing is the best way to counteract this.
Minimising DPD measurement error
Here is an easy to read, printable checklist to ensure accurate DPD readings every time.
Things to look out for
When was the last time your DPD was calibrated?
Like all measurement devices, handheld DPD colorimeters can drift over time, and need to be calibrated. Check your device manual for how often it should be calibrated, if you can’t remember the last time it was calibrated, chances are it needs doing again!
Stained Glass
The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
Tap water
If you use normal tap water to wash out vials, droplets left behind can affect your reading due to the residual chlorine in drinking water. It is best (but not always practical) to use deionized water to wash out your vials, but if this isn’t available (deionized water can be purchased as car battery top up water from any car parts supplier) then you can use cooled boiled tap water, as boiling gets rid of any chlorine. If not then simply make sure the vials are perfectly dry before use.
Little Known Chemistry
DPD has a wide range of interferents. This means recurrent problems can sometimes be caused by the chemical makeup of the sample. For example, chlorite (ClO2–) and chlorine dioxide both affect DPD, but only chlorine dioxide is measured by most chlorine dioxide amperometric sensors.
DPD can be used to track bromine, but DPD No.1 tablets measure FREE chlorine or TOTAL bromine. As combined bromine is just as effective a disinfectant as free bromine, this generally doesn’t pose too much of a problem, however some amperometric sensors measure free bromine, and cannot be calibrated using DPD No.1 tablets. For more information on measuring bromine, or chlorine in seawater, please see Pi’s technical note on Seawater Chlorination.
Rinse and repeat
How important is it to repeat my DPD measurement? Isn’t it a waste of time?
A sensor is only as good as its last calibration, and the sensor will be as accurate as you calibrate it to be. If you need your sensor for tight process control, such as a pool or dosing controller, then it is essential to repeat the DPD test at least twice, if not more. The reason it’s important to repeat the test is mainly due to human error, but variation in DPD tablets has been known, or it could be a slight concentration spike that you happened to pick up in your sample. With each repetition these circumstances become less and less likely, giving you more confidence in the value you use to calibrate your analyzer.
Pi recommends the following routine for calibration:
Perform a DPD test, and compare the reading to your analyzer.
- Is the reading within 10% of your analyzer? If yes, leave the analyzer alone.
- If the reading is not within 10%, repeat the DPD test.
- Is the second test within 10% of the first test? If yes, calibrate your instrument to this reading. If not, keep repeating the DPD tests until 2 consecutive tests are within 10%, then calibrate the machine to this reading.
Many different sites ranging across the whole water industry have a daily struggle to keep instrumentation functioning correctly due to fouling. However did you know that…
… self cleaning and self flushing systems are now available from Process Instruments for most types of sensors?
… these fouling removal systems can extend the life of sensors and drastically reduce maintenance regimes?
… Pi’s self cleaning/flushing systems are affordable, simple and trouble free by design?
Sensor Fouling – Why is it such a problem?
 Whatever the process being monitored is, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how regular should inspection and cleaning programs be for each piece of instrumentation? Too regular and the inspection and cleaning regime is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and probably fail prematurely.
Whatever the process being monitored is, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how regular should inspection and cleaning programs be for each piece of instrumentation? Too regular and the inspection and cleaning regime is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and probably fail prematurely.
Solution: Pi’s Autoclean & Autoflush Systems
Simple, reliable and easy to maintain, Process Instruments’ Autoclean/Autoflush systems are an alternative to mechanical cleaning mechanisms which can clog and break. By regularly spraying the sensor/probe with clean water or air, the sensor remains clean and free from fouling for extended periods of time. The sensor cleaning cycle is activated by Pi’s controller for a user selectable length of time and frequency so that no matter how dirty the application, the probe remains clean. With no moving parts in the sensor body or in the cleaning attachment there is nothing to replace or check other than a simple valve positioned in an easy to reach location.
Pi’s Autoclean and Autoflush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
Autoclean
This option can be added to our pH, ORP, Turbidity, Suspended Solids and Dissolved Oxygen (DO) sensors. Consisting of an end cap to direct the flow of clean water (or air for a DO sensor) across the face of the sensor blasting any dirt away. The cleaning is controlled by a single valve positioned in an easily accessible location.
Autoverify
If using air to clean a DO sensor the system can also automatically verify that the sensor is still responding correctly, removing any need to remove the sensor from the sample for months at a time.

Autoflush
For sensors that require flow cell mounting like Chlorine, Ozone and Chlorine Dioxide, an Autoflush system has inbuilt valves which automatically start/stop the sample flow and control the flow of clean water past the probe. The user can set the flushing interval and duration to keep the flow cell and sensor clear from fouling. For particularly dirty or stubborn contaminants, warm water can be used as the flush water to aid cleaning.
With the above options, whatever the application or parameter being measured, Process Instruments will be able to provide a monitoring system that will not only be accurate, precise and long lasting but that will also remain free from fouling and save the operator both time and money.

















































